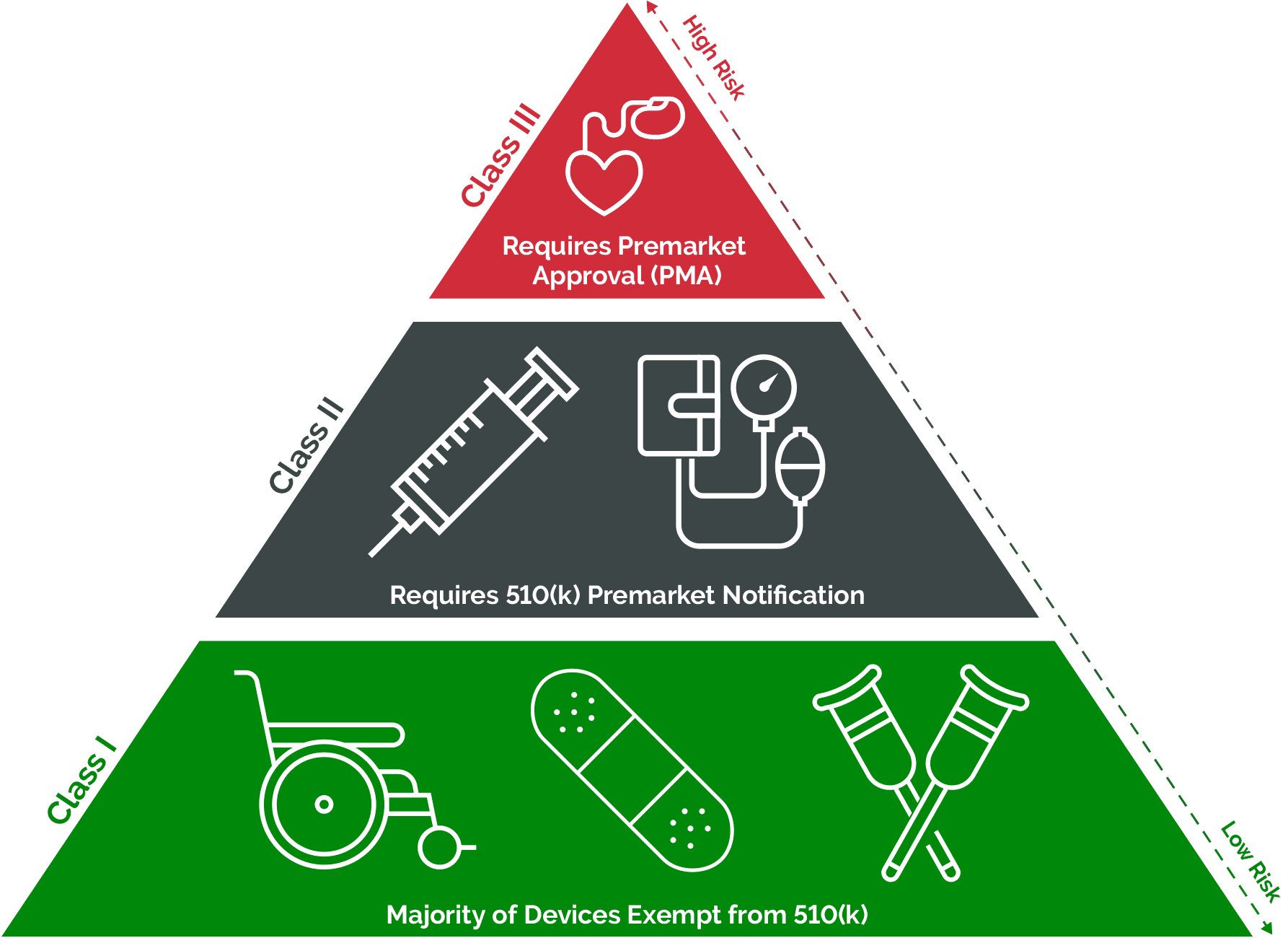
Medical Devices And Equipment Mean . — a medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or. the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. And monitors the safety of all regulated medical products. — what are medical devices? — the world health organisation (who) similarly defines medical devices as equipment whose intended primary mode of action is not. — medical devices are instruments, apparatuses, machines, or implants that are used for medical purposes. They are designed to diagnose,. according to article 1 of council directive 93/42/eec, [6] 'medical device' means any instrument, apparatus, appliance,. fda regulates the sale of medical device products in the u.s.
from www.arenasolutions.com
— medical devices are instruments, apparatuses, machines, or implants that are used for medical purposes. They are designed to diagnose,. — what are medical devices? An article, instrument, apparatus or machine that is used in the prevention, diagnosis or. — the world health organisation (who) similarly defines medical devices as equipment whose intended primary mode of action is not. according to article 1 of council directive 93/42/eec, [6] 'medical device' means any instrument, apparatus, appliance,. fda regulates the sale of medical device products in the u.s. the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. And monitors the safety of all regulated medical products. — a medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,.
How to Classify Your Medical Device for FDA Approval Arena
Medical Devices And Equipment Mean — the world health organisation (who) similarly defines medical devices as equipment whose intended primary mode of action is not. according to article 1 of council directive 93/42/eec, [6] 'medical device' means any instrument, apparatus, appliance,. — a medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or. fda regulates the sale of medical device products in the u.s. the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. They are designed to diagnose,. — what are medical devices? — the world health organisation (who) similarly defines medical devices as equipment whose intended primary mode of action is not. And monitors the safety of all regulated medical products. — medical devices are instruments, apparatuses, machines, or implants that are used for medical purposes.
From coastbiomed.com
UNDERSTANDING MEDICAL EQUIPMENT CLASSIFICATION Coast Biomedical Equipment Medical Devices And Equipment Mean according to article 1 of council directive 93/42/eec, [6] 'medical device' means any instrument, apparatus, appliance,. the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. — medical devices are instruments, apparatuses, machines, or implants that are used for medical purposes. And monitors the safety of all regulated medical products.. Medical Devices And Equipment Mean.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Symbol Use Medical Devices And Equipment Mean the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. And monitors the safety of all regulated medical products. fda regulates the sale of medical device products in the u.s. They are designed to diagnose,. — medical devices are instruments, apparatuses, machines, or implants that are used for medical purposes.. Medical Devices And Equipment Mean.
From medicaldevicereviews.weebly.com
Types of Medical Devices MEDICAL DEVICES Medical Devices And Equipment Mean An article, instrument, apparatus or machine that is used in the prevention, diagnosis or. — a medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. — the world health organisation (who) similarly defines medical devices as equipment whose intended primary mode of action is not. And monitors the safety of all. Medical Devices And Equipment Mean.
From whctllc.com
Surgical & Operating Room Solutions Western Health Care Technology Medical Devices And Equipment Mean fda regulates the sale of medical device products in the u.s. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or. according to article 1 of council directive 93/42/eec, [6] 'medical device' means any instrument, apparatus, appliance,. — what are medical devices? — a medical device can be any instrument, apparatus, implement,. Medical Devices And Equipment Mean.
From pactandpartners.com
Top 10 Medical Device Companies to Work For in 2020 Pact & Partners Medical Devices And Equipment Mean according to article 1 of council directive 93/42/eec, [6] 'medical device' means any instrument, apparatus, appliance,. And monitors the safety of all regulated medical products. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or. fda regulates the sale of medical device products in the u.s. — what are medical devices? —. Medical Devices And Equipment Mean.
From qbdgroup.com
What is a medical device? Key global definitions and regulations Medical Devices And Equipment Mean — a medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. — what are medical devices? And monitors the safety of all regulated medical products. fda regulates the sale of medical device products in the u.s. — medical devices are instruments, apparatuses, machines, or implants that are used for. Medical Devices And Equipment Mean.
From protoplastics.com
Medical Devices Equipment What Plastic Materials Are Used in Them? Medical Devices And Equipment Mean And monitors the safety of all regulated medical products. — what are medical devices? They are designed to diagnose,. the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. according to article 1 of council directive 93/42/eec, [6] 'medical device' means any instrument, apparatus, appliance,. — a medical device. Medical Devices And Equipment Mean.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Medical Devices And Equipment Mean according to article 1 of council directive 93/42/eec, [6] 'medical device' means any instrument, apparatus, appliance,. — a medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. fda regulates the sale of medical device products in the u.s. — medical devices are instruments, apparatuses, machines, or implants that are. Medical Devices And Equipment Mean.
From www.alamy.com
Surgical equipment and medical devices in operating room. Sterile scissors and other medical Medical Devices And Equipment Mean — the world health organisation (who) similarly defines medical devices as equipment whose intended primary mode of action is not. — a medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. And monitors the safety of all regulated medical products. They are designed to diagnose,. — what are medical devices?. Medical Devices And Equipment Mean.
From www.arenasolutions.com
Medical Device Definition Arena Medical Devices And Equipment Mean — what are medical devices? fda regulates the sale of medical device products in the u.s. — the world health organisation (who) similarly defines medical devices as equipment whose intended primary mode of action is not. — medical devices are instruments, apparatuses, machines, or implants that are used for medical purposes. according to article 1. Medical Devices And Equipment Mean.
From www.compliance-insight.com
The FDA Is Adopting ISO 13485 What This Means for Medical Devices Compliance Insight Medical Devices And Equipment Mean — medical devices are instruments, apparatuses, machines, or implants that are used for medical purposes. the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. They are designed to diagnose,. — what are medical devices? fda regulates the sale of medical device products in the u.s. — the. Medical Devices And Equipment Mean.
From remmed.com
FDA Registered Medical Devices What to Expect Remington Medical Devices And Equipment Mean — what are medical devices? according to article 1 of council directive 93/42/eec, [6] 'medical device' means any instrument, apparatus, appliance,. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or. — a medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. fda regulates the. Medical Devices And Equipment Mean.
From englishstudyonline.org
Medical Supplies Useful List of 30 Medical Equipment in English English Study Online Medical Devices And Equipment Mean An article, instrument, apparatus or machine that is used in the prevention, diagnosis or. — medical devices are instruments, apparatuses, machines, or implants that are used for medical purposes. — what are medical devices? — a medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. And monitors the safety of. Medical Devices And Equipment Mean.
From laegemiddelstyrelsen.dk
Medical devices Medical Devices And Equipment Mean They are designed to diagnose,. — the world health organisation (who) similarly defines medical devices as equipment whose intended primary mode of action is not. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or. fda regulates the sale of medical device products in the u.s. — what are medical devices? according. Medical Devices And Equipment Mean.
From angelanjohnson.com
Medical Devices Angela N Johnson Medical Devices And Equipment Mean — a medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. — the world health organisation (who) similarly defines medical devices as equipment whose intended primary mode of action is not. — medical devices are instruments, apparatuses, machines, or implants that are used for medical purposes. the food and. Medical Devices And Equipment Mean.
From www.mokomedtech.com
What is A Medical Device? MokoMedtech Professional OEM Medical Device Manufacturer Medical Devices And Equipment Mean — the world health organisation (who) similarly defines medical devices as equipment whose intended primary mode of action is not. — medical devices are instruments, apparatuses, machines, or implants that are used for medical purposes. according to article 1 of council directive 93/42/eec, [6] 'medical device' means any instrument, apparatus, appliance,. fda regulates the sale of. Medical Devices And Equipment Mean.
From thehealthcareinsights.com
Essential medical devices that should be available at your home The Healthcare Insights Medical Devices And Equipment Mean — a medical device can be any instrument, apparatus, implement, machine, appliance, implant, reagent for in vitro use,. — the world health organisation (who) similarly defines medical devices as equipment whose intended primary mode of action is not. — what are medical devices? according to article 1 of council directive 93/42/eec, [6] 'medical device' means any. Medical Devices And Equipment Mean.
From www.alamy.com
Medical equipment devices hires stock photography and images Alamy Medical Devices And Equipment Mean — medical devices are instruments, apparatuses, machines, or implants that are used for medical purposes. the food and drug administration (fda) has established classifications for approximately 1,700 different generic types of devices. An article, instrument, apparatus or machine that is used in the prevention, diagnosis or. — a medical device can be any instrument, apparatus, implement, machine,. Medical Devices And Equipment Mean.